IVT mRNA Synthesis
mRNA opens up novel therapeutic possibilities for various diseases.
Accelerated and simple mRNA manufacturing process creates greater potential for personalized medicines, such as individualized vaccines and protein replacement therapies for uncommon diseases.
Croyez offer a comprehensive solution for mRNA production that streamlines the entire workflow, from gene synthesis to IVT mRNA production.
Our expertise in in vitro transcription allows us to create customized mRNA molecules to suit your specific requirements.
We produce reliable mRNA aligned with your research goals, whether for therapeutic applications, functional studies, or other research needs.
✔️ Simplified and cell-free manufacturing
✔️ Independent of the cell cycle
✔️ No risk of insertional mutagenesis
Accelerated and simple mRNA manufacturing process creates greater potential for personalized medicines, such as individualized vaccines and protein replacement therapies for uncommon diseases.
Croyez offer a comprehensive solution for mRNA production that streamlines the entire workflow, from gene synthesis to IVT mRNA production.
Our expertise in in vitro transcription allows us to create customized mRNA molecules to suit your specific requirements.
We produce reliable mRNA aligned with your research goals, whether for therapeutic applications, functional studies, or other research needs.
✔️ Simplified and cell-free manufacturing
✔️ Independent of the cell cycle
✔️ No risk of insertional mutagenesis
Target Design
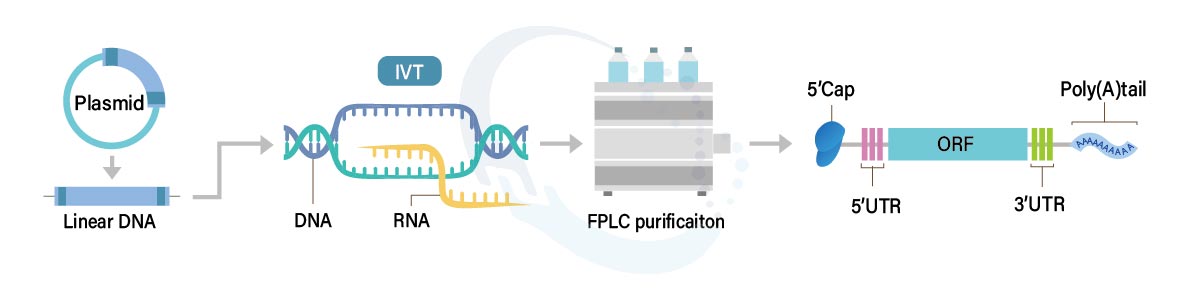
- Optimizing the sequence to attain optimal expression levels.
- 5’ and 3’ UTR
- Coding region
- Poly A tails of 80–125 nucleotides in length or longer are used in robust cloning and transcription methods to ensure high expression.
- Synthesis of mRNA ranging up to 10,000 nucleotides, scalable from microgram to milligram quantities.
- Achieving a high capping efficiency through enzymatic capping methods.
- Incorporating modified nucleotides for improved in vivo expression and decreased immune responses.
- Pseudouridine
- N1-Me-pUTP (m1Ψ)
- Proficient purification method by using FPLC swiftly and effectively removes impurities include
- IVT DNA template
- Residual protein
- Double-stranded RNA (dsRNA)
- Gene synthesis: depends
- IVT mRNA synthesis: 2-3 weeks
Provided by Customers
- Target sequence or plasmid with the plasmid map
- Requirements form
- mRNA final products
- Testing report
Croyez provides a wide range of quality control (QC) methods for IVT mRNA. Standard QC items are consistently carried out, while optional QC measures are executed based on specific project requirements.
.png)
.png)


